Extending the life of tools: how you can remove rust from metal surfaces
During the operation of metal products, a moment inevitably comes when corrosion appears on the metal surface. In other words, the product begins to rust, which reduces its performance and eventually makes it completely unusable. To prevent this from happening, the homemaster.techinfolux.com/en/ editors have prepared a number of recommendations on how to remove rust from a metal surface using available methods.
The content of the article
- 1 How does corrosion affect the operation of equipment and tools
- 2 How to remove rust from metal: machining
- 3 How to remove rust from a metal surface using heat treatment
- 4 What chemical agents are easier to wash off rust from metal
- 5 Folk remedies for removing rust from metal
- 6 What unusual means and methods to remove rust from metal
How does corrosion affect the operation of equipment and tools
What is metal corrosion? As people of the older generation would say, this is such a hellish foreign band that destroys our brain with their music. But let's not argue about tastes and leave the group "Metal corrosion" alone, but the essence of the statement is correct: it is a chemical process that destroys a metal surface.

Equipment and tools made of metal and subjected to a corrosive process begin to change outwardly. At first it is almost invisible to the naked eye. Then rusty ulcerations and roughness appear on the surface.
The process has started, and if it is not stopped, damage will penetrate deep into the material. All metal devices damaged by rust start counting until they are written off. Therefore, to prevent this from happening, you need to remove the corrosion in time.
How to remove rust from metal: machining
Mechanical processing is the first thing that comes to mind when the question arises of how to remove rust from metal.As a mechanical method, drill bits, a sander, files, sandpaper of various sizes are used.
How to remove rust from a metal surface using heat treatment
During heat treatment, the damage is exposed to high temperatures, often combined with air or water flow.
The hot air stream will remove rust stains by progressively softening and crushing the scale.
What chemical agents are easier to wash off rust from metal
The first thing that comes to mind is a rust converter, but this action will only suspend the corrosion, not remove it.
In order not to waste money and time, it is better to immediately start acting effectively and use chemicals.
How to get rid of rust on metal with zinc chloride
Metal cleaning from rust can be done with zinc chloride and tartar (potassium salt of tartaric acid) in a ratio of 5 g and 0.5 g. Substances are diluted in 100 ml of water. The acidity will be increased, and rust will lose ground.
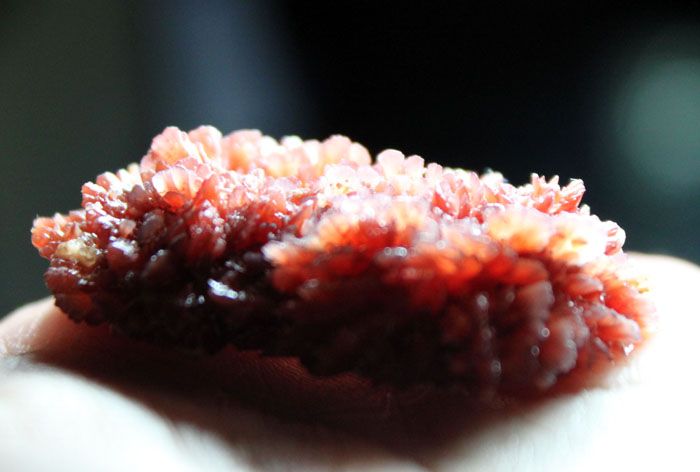
Tartar - a precipitate in the form of crystals formed as a result of the production of wine during alcoholic fermentation
Processing should be done with protective equipment on hands and with open windows.
Hydrochloric or sulfuric acid
The most well-known fluids for removing rust from metal are hydrochloric (hydrochloric) or sulfuric acid. Acids are used in conjunction with inhibitors.
Rust will be reduced if it is moistened with an aqueous solution of one of the proposed acids and urotropin. Urotropine is the very inhibitor that will inhibit the corrosion process. Why can't you work without this substance? Because acid is an aggressive thing, and without an inhibitor it can damage metal during processing.
Small metal parts are simply immersed in a 5% solution of urotropine acid (at the rate of 0.5 g per 1 liter). Larger fragments are processed with a brush.
Advice! Rust-locked nut and bolt will easily move away from each other if you take plasticine and make a side around the nut out of it. It is filled with a sulfuric solution with the addition of zinc.
Oxalic acid
It is the simplest strong organic acid, first synthesized in 1824. They work with her exclusively in rubber gloves, glasses and a robe. It is very dangerous to inhale harmful vapors!
An object affected by rust is washed under water with the addition of dishwashing liquid, wiped dry and dipped in the resulting solution for 20-25 minutes. Can be supplemented with mechanical brushing.

Rust eats up small objects very quickly, so do not wait, but rather start the cleaning procedure right now
After cleaning, the item is rinsed with running water and dried.
Lactic acid
One thing that eats away rust well is lactic acid.
The following composition will help remove overgrown rust: 90-100 g of vaseline oil (can be replaced with the same amount of liquid paraffin) and 50 g of lactic acid.
The cleaning process looks like this: corrosion turns into salts, which dissolve in a paraffinic liquid. Finally, the already cleaned object is wiped with a cloth smeared with petroleum jelly.
Folk remedies for removing rust from metal
In addition to chemical poisonous substances, there are equally powerful folk remedies for removing rust from metal.Traditional cleansers are potatoes, soda, citric acid, and vinegar.
How to get rid of rust on metal with potatoes
For many, this may seem unexpected, but people have long understood that such an ordinary potato in everyday life will be able to cope with a bang with traces of corrosion.
You will need one potato and laundry soap: the tuber is cut into two parts and the cut is soaped, which will help to cope with the rusty surface. It is best to leave the potatoes in the damaged area for 3-4 hours.
During this time, you can trim the same tuber, lather the cut again and put the potato on a rusty place.And some people also prepare a special liquid for removing rust from metal from potato tops - it becomes a kind of inhibitor.
All greens should be placed in a 3-liter jar - half. Add hydrochloric or hydrochloric acid to it so that it slightly covers the tops. For 15-20 minutes we feel like sorcerers, stirring the potion. And then the liquid is drained into another container: you can use it for anti-corrosion treatment.
How to remove rust from metal at home with citric acid or lemon juice
Packaging citric acid it costs literally a penny, you can buy this miracle in any grocery store or supermarket in the "spices" or "everything for baking" section.
One package is poured into a container and poured with such an amount of water that will allow the whole object to be cleaned to be immersed in it. The indicator of the reaction will be bubbles rising to the surface. The container is left untouched for 12-13 hours. Then the object is taken out of the solution, rinsed and dried.
Rusty spots are sprinkled with salt and juice is squeezed out of the fruit. There is no need to save: the more the surface is wetted, the better the result will be. It is better to leave the object soaked for 3 hours, and then peel it with a lime or lemon peel.
How to clean rust from metal with soda
Soda has become so firmly established in our everyday life that funny memes and jokes about it are circulating on the Internet.
This time, the household assistant will show his superhero power not in the kitchen, but in the fight against rust corroding metal products. All you need to do is stir the soda with a little water until a slurry is formed. The mixture is smeared over the damaged area and after a while everything is brushed off with an old toothbrush.
Vinegar as a rust remover for metal
Vinegar is also on the shelf in almost every kitchen. It simply eats away the rust itself, therefore it is an excellent anti-corrosion agent.
White vinegar reacts well with corrosion, removing all rusty marks on metal. You can simply dip the item in vinegar and leave it there for 2-4 hours. The rusty paste is simply scraped off, and the item is rinsed and dried.
What to do if the item turns out to be large: vinegar is poured into a flat container like a baking sheet - you still have to pickle the item. If the damage to the metal is not severe, it is easier to simply soak a cloth in vinegar and moisten the object properly.
Advice! Vinegar and aluminum foil as a grater work especially well and effectively.
Hydrogen peroxide against corrosion
Another working remedy for rust stains.
Peroxide is poured directly from the bottle onto the affected area. After a while, a rusty gruel appears on the surface of the metal product. It is simply brushed off.
If borax is included in the composition, then this union will corrode any rust.
What unusual means and methods to remove rust from metal
The list of working anti-corrosion compounds is not complete. There is also the beloved by many Coca-Cola, tomatoes and the electrolysis process.
Coca Cola
Glory to the American meticulous housewives, who were the first to notice how a delicious drink can eat away iron oxide. And not only the cola eats the rust, but the nail itself. There is nothing surprising in this, grapefruit works in much the same way: both Coca-Cola and the fruit contain phosphoric acid.

How to remove rust from a bike? Or how to clean a rusted hammer? Buy a cola, and thoroughly bathe the item in it!
It is important not to miss the moment when the object has cleared, and the drink has not begun to corrode the metal itself.
Saving metal from the garden or from the kitchen: tomato paste or ketchup
The chemical composition of tomato makes it possible to use it not only for food.
They drip on rusty metal with ketchup or paste, but do not erase it right away: let the substances interact. After 10-15 minutes, dirt is removed mechanically, and the surface is wiped off with a dry cloth.
Electrolysis
There is also a longer, but more effective way to clean metal products.
For cleaning, you need a container commensurate with the items, a steel plate for the anode, caustic soda or "Whiteness", long stranded copper wires (insulated), a battery, a plastic plate (like a screen that protects against contact with the anode), a metal brush and rags.
Attention! The process is quite risky, since if the amperage exceeds the norm for the selected volume of water, the mixture will begin to boil. It is better to carry out work outdoors or in a well-ventilated area.
- A solution is created: caustic soda (half a glass) for 15 liters of water.
- The anode is made from a wire, installing it in the container in such a way that it does not touch its walls.
- We lower the round electrode (or steel plate) into the container. Aluminum is not used! The length of the anode must be sufficient so that part of it is above the surface of the solution.
- With a crocodile clip, a wire is attached to the anode, directed to the positive red terminal (from the battery).
- A steel plate is lowered into the container, making sure that it protrudes by a third from the solution.
- The end of the second wire is attached to the soiled object. One end of the wire is cleaned of insulation, the veins are stripped and this end is attached to the object. At the point of contact, rust must be removed mechanically.
Important! There should be no free end of the protected wiring that could touch the anode.
It remains to attach the second wire to the black terminal. The process will take several hours and the solution will turn dirty brown. The cleaned parts can become dark - such plaque is removed with a brush.
The more carefully you treat metal products, the longer they will last. We offer you to watch a video of the electrolysis process at home: